Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (2): 291-297.doi: 10.3969/j.issn.2095-4344.0653
Previous Articles Next Articles
Effects of stress on degradation of biodegradable metal for vascular stent application: a review
Lu Yun1, Gu Xuenan1, 2, Fan Yubo1, 2
- 1School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China; 2Beijing Advanced Innovation Center for Biomedical Engineering, Beihang University, Beijing 100083, China
-
Received:2018-07-03Online:2019-01-18Published:2019-01-18 -
Contact:Gu Xuenan, PhD, Associate professor, School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China; Beijing Advanced Innovation Center for Biomedical Engineering, Beihang University, Beijing 100083, China -
About author:Lu Yun, Master candidate, School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China -
Supported by:the National Natural Science Foundation of China, No. 51401007 (to GXN); the Foundation for the Author of National Excellent Doctoral Dissertation of China, No. 201463 (to GXN); Young Elite Scientists Sponsorship Program by CAST, No. 2017QNRC001 (to GXN)
CLC Number:
Cite this article
Lu Yun, Gu Xuenan, Fan Yubo. Effects of stress on degradation of biodegradable metal for vascular stent application: a review[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(2): 291-297.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
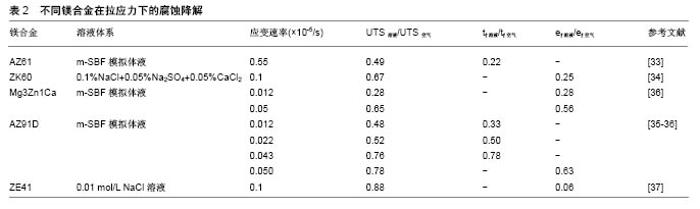
2.1 应力对镁合金降解的影响 镁合金是目前研究最多的可降解金属材料,它作为血管支架材料具有以下优势:①镁及其合金具有较高的力学强度:WE43镁合金的抗拉强度达到250 MPa,屈服强度为162 MPa,弹性模量为45 GPa,可为血管提供足够的径向支撑力。以镁合金为基材的AMS-II可降解支架,塌缩压力达到 150 kPa,与不锈钢相当[15];②生物降解性:镁的标准电极电位很低,化学性质十分活泼,在体液中容易发生化学反应,形成镁离子,被人体吸收;③生物相容性:镁是人体细胞第2重要阳离子,生物相容性好,具有低致栓性和低炎症反应[20-21],对人体新陈代谢至关重要[22]。镁能激活体内多种酶,维持核酸的稳定性,参与体内蛋白质的合成、肌肉收缩及体温调节[23-24]。 镁的标准电极电位很低,E0= -2.372 V,在水溶液中极易发生如下反应[25-26]: (1) (2) (3) Mg→Mg2++2e-(阳极反应) 2H2O+2e-→2OH-+H2 (阴极反应) Mg+2OH-→Mg(OH) 2(沉积产物) 从上述反应方程式可看出,镁及镁合金与水发生了电化学反应,生成反应物Mg(OH)2和氢气,Mg(OH)2覆盖在镁合金表面形成保护膜,可以减缓镁的降解。 Mg(OH) 2+2Cl-→Mg2++2OH-+2Cl- 但是,人体内富含Cl-,Mg(OH)2保护层会受到氯离子的侵蚀而发生溶解,具体反应过程如下: (4) 以上反应导致了Mg(OH)2的破裂,而Cl-又能够进一步通过保护膜破裂后的缝隙与Mg基体发生反应,引起更严重的点蚀。 镁合金在腐蚀环境中降解速度很快,特别是在应力的共同作用下[27-30],因此,明确镁合金上在拉应力、压应力、剪切应力和周期性循环应力等不同应力模式作用下的降解尤为重要。 2.1.1 镁合金的应力腐蚀开裂 Jafari等[31]首先采用慢应变拉伸法评价了MgZn1Ca0.3镁合金的应力腐蚀,应变速率分别为3.1×10-7/s,腐蚀环境是空气和m-SBF模拟体液,MgZn1Ca0.3镁合金的热处理温度分别为 325 ℃和400 ℃。结果显示不同处理温度下的2种MgZn1Ca0.3镁合金应力腐蚀敏感,在m-SBF模拟体液中的延伸率仅为空气中的30%,在m-SBF模拟体液中抗拉强度降低;在325 ℃下的m-SBF模拟体液中,MgZn1Ca0.3镁合金的抗拉强度为空气中的96%;在400 ℃下的m-SBF模拟体液中,MgZn1Ca0.3镁合金的抗拉强度为空气中的75%。但值得注意的是,m-SBF模拟体液中2种MgZn1Ca0.3镁合金的弹性极限和屈服强度与空气中的数值相同。 Koo等[32]采用慢应变拉伸法评价AZ31B镁合金和ZE41镁合金在模拟体液中的应力腐蚀开裂,测试时间为30 d和90 d。结果表明,AZ31B镁合金和ZE41镁合金在长时间的慢应变拉伸中均表现出应力腐蚀敏感,而且AZ31B镁合金的应力腐蚀性更强,机械强度下降更快。 Törne等[33]采用慢应变拉伸法评价AZ61镁合金的应力腐蚀,应变速率为5.5×10-6 mm/s,腐蚀环境分别为空气、m-SBF模拟体液。结果显示AZ61镁合金应力腐蚀敏感,在m-SBF中的抗拉强度仅为空气中的49%,断裂时间仅为空气状态下的22%。 Zhou等[34]发现当应变速率为1×10-6/s时,ZK60镁合金在模拟体液中的应力腐蚀敏感性高于空气,抗拉强度、屈服强度、延伸率下降很快,其中抗拉强度为空气中的67%,认为ZK60出现应力腐蚀开裂的主要原因是:大量氢气在应力作用下聚集在开裂尖端处,引发支架断裂,符合氢致脆化理论。 Choudhary等[35]首先采用慢应变拉伸法评价了AZ91D镁合金的应力腐蚀,应变速率分别为0.012× 10-5/s、0.022×10-5/s、0.043×10-5/s,腐蚀环境是空气和m-SBF模拟体液。结果表明,AZ91D镁合金在m-SBF中的应力腐蚀敏感性高,并且拉伸速率显著影响应力腐蚀。在应变速率为0.012×10-5/s时下降程度最低,分别为空气中的48%和33%。Choudhary等[36]对比了Mg3Zn1Ca和AZ91D两种镁合金的应力腐蚀,当应变速率为1.2×10-7/s、5×10-7/s时,两种镁合金均应力腐蚀敏感,并且应变速率越低,敏感性增强。当应变速率为5× 10-7/s时,Mg3Zn1Ca和AZ91D镁合金的抗拉强度分别变为空气环境下的65%,78%,延伸率仅为空气下的56%和63%,Mg3Zn1Ca镁合金的应力腐蚀敏感性更强。 宋仁国等[37]指出ZE41镁合金在NaCl溶液中具备一定的应力腐蚀敏感性,抗拉强度和延伸率均比空气中的低,分别为空气中的88%和6%,且板材厚度也对ZE41应力腐蚀敏感性有影响,一般而言,厚试样的应力腐蚀敏感性高于薄试样。 王璐科等[38]采用慢应变拉伸法评价了AZ91D镁合金的应力腐蚀,当应变速率分别为1.67×10-5/s、4.17×10-5/s、8.33×10-5/s时,腐蚀环境为空气、NaCl溶液、NaNO3溶液。结果表明AZ91D镁合金的腐蚀具有明显的离子选择性,AZ91D镁合金不受外力作用时,在含有Cl-的腐蚀溶液中腐蚀严重;AZ91D镁合金在受到外力作用时,在含有NO3-的腐蚀溶液中腐蚀更严重。应变速率也是影响AZ91D镁合金应力腐蚀的一个重要因素,AZ91D镁合金在受到给定的3组应力作用时,均表现出显著的应力腐蚀敏感性,且速率在4.17×10-5/s时应力腐蚀最严重。不同镁合金在拉应力作用下的腐蚀降解,见表2。 例如,Mg3Zn1Ca镁合金在SBF模拟体液中的应力腐蚀敏感性高于AZ61镁合金和AZ91D镁合金,此外应力腐蚀对镁合金断裂延伸率影响显著,与在空气中相比,其断裂延伸率下降37%-94%,表明应力腐蚀开裂对于镁合金支架十分危险,严重影响镁合金血管支架服役周期及临床治疗效果。应力腐蚀开裂是指材料在拉应力和一种特定腐蚀介质共同作用下发生的破裂。镁合金的应力腐蚀开裂机制主要有2种:阳极溶解和氢致脆化。 "
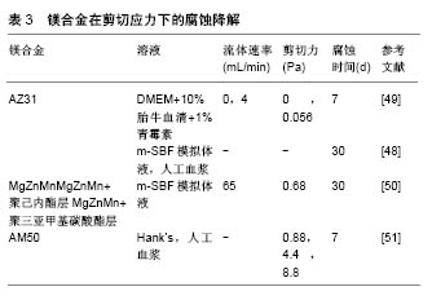
阳极溶解理论认为镁合金应力腐蚀主要包括3个过程:首先是电化学作用导致局部膜破裂,然后是开裂尖端定向溶解,引发裂纹进一步扩展,最后裂纹到达临界尺寸,发生失稳断裂[39]。氢致脆化理论认为镁合金在腐蚀溶液中最初会发生析氢腐蚀[40],产生的大量氢气会在应力作用下形成氢浓度梯度,大量集中在在开裂尖端处,当氢气浓度在应变作用下达到临界值,氢化物就会在开裂尖端沉淀,最终诱发脆变[41-42],导致镁合金开裂。 2.1.2 压应力下的镁合金降解 许多研究表明,压应力影响镁合金腐蚀降解。Yang等[43]研究了挤压态Mg-5Ca和Mg-5Ca-Zn镁合金在压应力下的腐蚀降解,应变速率为10-3/s,腐蚀环境为Hank’s模拟体液。研究结果显示,Mg-5Ca-Zn镁合金的抗压强度和屈服强度均高于Mg-5Ca镁合金,而且Mg-5Ca-Zn镁合金在Hank’s模拟体液浸泡30 d后的力学性质并未明显下降,抗压强度仅由385 MPa下降为360 MPa。 Dong等[44]讨论了30-90 N压力下AZ31镁合金的腐蚀降解,研究显示加载30 N的压应力,AZ31镁合金失重约30%,加载60 N时失重约40%,加载90 N时失重约60%。结果表明压应力明显加快AZ31镁合金在腐蚀环境下的降解,而且随着压应力的增加,AZ31镁合金腐蚀程度越来越大。 Zheng等[45]研究了挤压态Mg-2.65Zn镁合金在压应力下的腐蚀降解,应变速率为4.17×10-4/s,腐蚀环境为Hank’s模拟体液。研究结果显示Mg-5Ca-Zn镁合金在压力作用下的质量损失显著高于无应力作用下的质量损失,说明镁合金在压应力的作用下降解明显加快。 Denkena等[46]研究了LAE442镁合金在5 kN压应力下的腐蚀降解,腐蚀环境为0.9%NaCl。研究表明处于 5 kN压应力作用下,LAE442镁合金析氢量比无应力状态增加3倍以上,表明镁合金在压应力的作用下降解明显加快。并且LAE442镁合金在拉应力和压应力复合作用时的降解比单一应力作用时的降解快。 一般来说,压应力加快镁合金的降解机制主要有2种:①压应力导致的镁合金发生变形,合金内部产生变形孪晶,导致降解加速[45];②降解过程中镁合金表面形成氢氧化物层,但它并不稳定,外部应力的作用会破坏表层并促进层中微裂纹的扩展,最终暴露镁基体,镁基体和氧化层之间的电位差形成腐蚀微电池,最终导致降解。这就说明压应力可通过影响表面腐蚀产物层及改变合金显微组织2个层面影响镁合金腐蚀降。 2.1.3 剪切应力下的镁合金降解 镁合金支架植入血管后会受到因血液流动冲刷产生的剪切力作用,这会在一定程度上影响支架的降解。Wang等[47]发现高纯Mg和AZ31镁合金在流动介质中的腐蚀降解速率均明显高于静止介质,但文章并未给出具体流速与剪切力数值。 劳永华等[48]研究了AZ31镁合金在人工血浆中的动态降解行为,发现加载流动的腐蚀溶液环境可加速AZ31镁合金的腐蚀,当腐蚀时间达到30 d时,流动腐蚀环境下材料的析氢量是静止环境中的2倍,但研究并未给出具体剪切应力数值。 Wang等[49-50]研究不同剪切应力作用下AZ31镁合金支架的降解,研究选择的剪切应力分别为0 Pa和0.056 Pa。结果表明随着剪切应力的增加,AZ31镁合金从均匀腐蚀向丝状腐蚀转变,当剪切应力达到0.056 Pa时,可观察到由于腐蚀产物剥离造成的局部腐蚀产物膜开裂情况,且随着时间的推移而腐蚀加剧,此时支架的体积损失率达到了未加剪切力时的2倍。而且研究还表明镁合金腐蚀方向受流体冲刷方向的影响。Wang还对比讨论了MgZnMn镁合金和表面改性后的MgZnMn镁合金在相同剪切力条件下的降解行为,剪切力为 0.68 Pa。结果表明,表面改性后MgZnMn镁合金的抗剪切力作用明显增强,Mg离子析出浓度和失重均低于MgZnMn镁合金。表面改性方式不同的镁合金抗剪切力作用不同,其中聚三亚甲基碳酸酯涂层改性MgZnMn镁合金样品的抗剪切力效果优于聚己内酯涂层改性MgZnMn镁合金。 Lévesque等[51]模拟了心血管装置中镁合金的降解实验。实验结果显示镁合金的腐蚀速率和腐蚀机制随着镁合金所受到的剪切力不同而改变。当剪切力在 0.88 Pa和4.4 Pa时,AM50镁合金降解速度比不受到剪切力作用时低,但当剪切力增加到8.8 Pa时降解速率增加了近50%。 镁合金在剪切力的作用下降解加快,并且其降解速度随着剪切力的增加而增加。剪切力不仅改变了镁合金的降解速率,还会改变其腐蚀机制,在低剪切力时,镁合金主要为均匀腐蚀,随着剪切力的增加,样品表面趋向于局部腐蚀。剪切应力加速镁合金腐蚀的机制可能是:随着剪切应力增大,腐蚀溶液中离子扩散增加,影响镁与腐蚀介质界面的化学平衡,使腐蚀增强。此外,降解速率还与腐蚀产物的去除相关。随着剪切力的增加,腐蚀溶液流动速率增加,样品表面的腐蚀层逐渐脱 落,镁基体暴露腐蚀加快。不同镁合金在剪切应力作用下的腐蚀降解,见表3。"
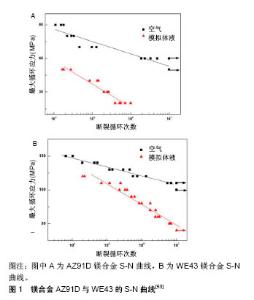
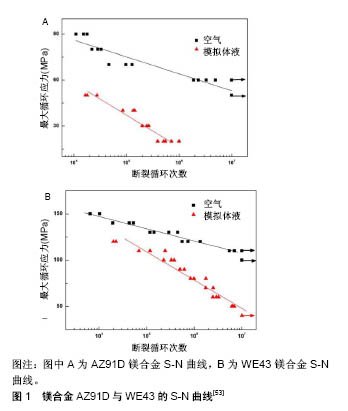
2.1.4 镁合金的腐蚀疲劳 Jafaria等[52]研究AZ91D合金在m-SBF模拟体液中的腐蚀疲劳行为,结果显示其可在m-SBF模拟体液中循环106次,疲劳强度由90 MPa下降至20 MPa。表明在循环荷载作用下,镁合金的疲劳强度显著下降。 Gu等[53]研究了AZ91D和WE43镁合金在循环荷载下的腐蚀疲劳行为,腐蚀环境分别为空气和SBF模拟体液,见图1。结果显示在循环加载条件下,2种实验合金的腐蚀速率比静态合金的腐蚀速率要高。AZ91D镁合金在空气中循环107次,疲劳强度下降至50 MPa。AZ91D镁合金在SBF模拟体液中循环106次,疲劳强度下降至20 MPa。WE43镁合金在空气中循环107次,疲劳强度下降至110 MPa。WE43镁合金在SBF模拟体液中循环107次,疲劳强度下降至40 MPa。通过微观分析表明镁合金在空气中的疲劳裂纹源于微孔,而在SBF模拟体液中的疲劳裂纹源于腐蚀坑。WE43为韧性断裂,而AZ91D为脆性断裂。"
Bian等[54]研究了高纯镁(99.99%)和Mg-1Ca、Mg-2Zn-0.2Ca三种镁合金在循环荷载下的腐蚀疲劳行为,腐蚀环境分别为空气和SBF模拟体液,疲劳循环为4×106次。结果显示3种材料在空气中的疲劳强度都在 90 MPa左右,而浸泡SBF模拟体液中后,疲劳强度分别下降为52,70,68 MPa。研究还对比了镁及镁合金处于SBF模拟体液静态介质和SBF模拟体液循环荷载环境下的降解速率,结果显示在后者中的降解速率显著高于前者,证明循环荷载加快材料在腐蚀环境下的降解速率。 血管支架的疲劳断裂是其临床治疗失败的主要原因之一。在腐蚀环境中镁合金疲劳强度下降显著,影响镁合金循环荷载腐蚀疲劳行为的因素主要有以下几个:加载频率、加载模式、加载载荷,其中加载模式是指加载荷载的类型,当施加在镁合金基体的应力是多向复合应力时,其降解速率会明显高于单向拉伸或压缩荷载作用。腐蚀疲劳实验实际是拉应力、压应力、剪切应力的综合,它能较好地模拟植入物在体内经受的复杂多轴载荷,了解不同类型荷载作用下材料开裂的位置与程度,对预测其在体内的服役周期及断裂机制具有一定的辅助作用。 (5) (6) (7) (阳极反应) (阴极反应) (沉积产物) (5) (6) (7) 2.2 应力对铁降解的影响 铁是人体必需的微量元素,也是血红蛋白的重要组成部分。铁可增加中性白细胞和吞噬细胞的吞噬能力,提高机体的免疫能力[55-56]。纯铁的韧性较好,力学性能优于镁合金,与316L不锈钢接近,纯铁的抗拉强度为180-210 MPa,屈服强度为120- 150 MPa。先健科技有限公司研发的注氮铁支架,支架梁厚度可以达到70 μm,回弹为2.21%,支架最大截面尺寸为0.99 mm,径向支撑力为171 kPa[57]。但铁的降解速率较慢,远小于镁的降解速率,Fe的标准电极电位为:E0=-0.447 V,在体内发生的电化学反应方程为[58]: Fe→Fe2++2e-(阳极反应) O2+H2O+2e-→2OH-(阴极反应) Fe2++2OH-→Fe(OH) 2(沉积产物) (8) (9) Fe在腐蚀溶液中首先氧化为Fe2+,Fe2+直接和氧作用形成氢氧化亚铁沉淀,在Fe表面形成一层致密的氧化层,阻碍Fe的进一步降解,而当腐蚀溶液呈碱性且氧气充足时,一部分Fe2+会进一步氧化成Fe3+,并形成更致密的Fe(OH)3保护层,具体的反应如下: Fe2+→Fe3++ e- Fe3++3OH-→Fe(OH)3 (10) 但当人体的Cl-达到一定浓度时,会使Fe(OH)3水解为2FO(OH),而水解产物继续与Fe(OH)2反应形成Fe3O4,具体的反应如下: Fe(OH)2+2FO(OH)→Fe3O4+H2O 生成的Fe3O4不溶于水,和Fe(OH)3、Fe(OH)2一起构成Fe的保护层,阻碍Fe的进一步腐蚀,因此Fe在可降解金属血管支架材料中降解速度最慢。 Zhu等[59]研究了纯铁在剪切力条件下的降解行为,剪切应力大小为1.14 Pa,腐蚀环境为SBF模拟体液。结果表明随着浸泡时间的增加,纯铁的平均质量由638.6 mg降为613.8 mg,平均降解速率为20.4 µg/cm3,高于静态介质中的降解速率。浸泡1个月后,纯铁呈现出均匀腐蚀,且能够在较长时间里维持机械完整性。 Liu等[60]研究了纯铁在剪切力条件下的降解行为,剪切力为0.68 Pa,腐蚀环境为Hank’s溶液,浸泡时间为 30 d。结果显示剪切腐蚀最先出现在纯铁试样边缘位置,逐渐向中心移动,且腐蚀方向就是流体冲刷的方向。结果还表明处于剪切力作用下纯铁的降解速率显著高于无应力状态下纯铁的降解速率,这可能是由于流动腐蚀环境可抑制铁离子局部的累积,及时冲刷了铁的腐蚀产物保护层。文章并没有给出这两种状态下降解速率的具体比值。 (11) (12) (13) 2.3 应力对锌降解的影响 锌是人体必需微量元素,它参与人体80多种酶的代谢过程,是人体生长发育生殖遗传必不可少的元素[61-63]。纯锌既有类似于镁合金的生物相容性又不易受外界磁场的干扰。纯锌的抗拉强度远低于镁合金和纯铁,大约为50 MPa,但它具有很好的延展性。在锌中添加少量合金化元素,能够显著改善其拉伸强度,见图2[64],Zn的标准氢电极电位:E0=-0.763V,介于Mg(-2.372 V)与Fe(-0.447 V)之间,降解速率介于镁合金和纯铁之间,因此锌也成为可降解金属支架材料研究的热点。 锌在水溶液中发生反应生成氢氧化锌,整个过程不产生氢气,其反应过程如下[65]: Zn→Zn2++2e- 2H2O+O2+4e-→4OH- 2Zn+O2+2H2O→2Zn(OH)2 (14) 生成的反应产物Zn(OH)2不溶于水,会形成保护膜抑制Zn的进一步腐蚀,使得材料植入初期降解过程逐渐减慢。但随着浸泡时间的增加,当溶液中的Cl-达到一定浓度后会使不溶于水的Zn(OH)2溶解,反应方程如下: 这表明Zn的表面会在氯离子的作用下逐渐侵蚀,发生局部腐蚀。但人体的生理环境十分复杂,还包括蛋白质、氨基酸有机分子及Na+等离子。降解的锌离子最终和溶液中的HPO4-2、Na+或K+生成不溶于水的磷酸盐。 (15) (16) 国内外关于应力对锌的降解影响方面的文献较少。Törne等[33]采用慢应变拉伸法评价了纯锌的应力腐蚀,应变速率为3.2×10-5 mm/s,腐蚀环境分别为空气、m-SBF模拟体液和全血。结果显示纯锌的应力腐蚀敏感性比AZ61的应力腐蚀敏感性差很多,其在m-SBF浸泡后的抗拉强度和在空气下的抗拉强度大致相同,断裂时间也只比后者降低了25%。纯锌在全血中应力腐蚀不敏感,力学性能和空气中测试的结果接近,甚至没有明显裂纹。 "
| [1] Moravej M,Mantovani D.Biodegradable metals for cardiovascular stent application: interests and new opportunities.Int J Mol Sci. 2011;12(7): 4250-4270.[2] Li N,Zheng Y.Novel magnesium alloys developed for biomedical application: a review. J Mater Sci Technol. 2013;29(6):489-502.[3] Zartner P, Cesnjevar R,Singer H,et al.First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby.Catheter Cardiovasc Interv. 2005;66(4):590-594.[4] 杜博,赵学忠.冠心病患者支架内再狭窄的危险因素[J].中国老年学杂志, 2015,9(10):2708-2710.[5] Niinomi M,Nakai M,Hieda J.Development of new metallic alloys for biomedical applications. Acta Biomaterialia. 2012;8(11):3888-3903.[6] Staiger MP,Pietak AM,Huadmai J,et al.Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials.2006;27(9):1728-1734.[7] Garza L, Aude YW,Saucedo JF.Can we prevent in-stent restenosis? Curr Opini Cardiol.2002;17(5):518.[8] Seitz JM,Durisin M,Goldman J,et al.Recent advances in biodegradable metals for medical sutures: a critical review. Adv Healthc Mater. 2015; 4(13):1915-1936.[9] Francis A,Yang Y,Virtanen S,et al.Iron and iron-based alloys for temporary cardiovascular applications.J Mater Sci Mater Med. 2015; 26(3):138.[10] Tsuji T,Tamai H,Igaki K,et al.Biodegradable polymeric stents.Curr Interv Cardiol Rep.2001;3(1): 10-17.[11] Ramcharitar S,Serruys PW.Fully biodegradable coronary stents. Am J Cardiovasc Drugs.2008;8(5):305-314.[12] Meng B,Wang J,Zhu N,et al.Study of biodegradable and self-expandable PLLA helical biliary stent in vivo and in vitro. J Mater Sci Mater Med.2006;17(7):611-617.[13] Zhu X,Braatz RD. A mechanistic model for drug release in PLGA biodegradable stent coatings coupled with polymer degradation and erosion.J Biomed Mater Res A. 2015;103(7):2269-2279.[14] Ma E,Xu J.Biodegradable alloys: the glass window of opportunities.Nat Mater.2009;8(11):855.[15] Im SH,Jung Y,Kim SH.Current status and future direction of biodegradable metallic and polymeric vascular scaffolds for next-generation stents. Acta Biomaterialia. 2017;60(1):3-22.[16] LaDisa JF,Guler I,Olson LE,et al. Three-dimensional computational fluid dynamics modeling of alterations in coronary wall shear stress produced by stent implantation. Ann Biomed Eng.2003;31(8):972-980.[17] Carlier SG,van Damme LCA,Blommerde CP,et al. Augmentation of wall shear stress inhibits neointimal hyperplasia after stent implantation: inhibition through reduction of inflammation? Circulation. 2003;107(21): 2741-2746.[18] Peuster M,Hesse C,Schloo T,et al.Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials.2006’27(28):4955-4962.[19] Guillory RJ,Bowen PK,Hopkins SP,et al.Corrosion characteristics dictate the long-term inflammatory profile of degradable zinc arterial implants. ACS Biomater Sci Eng. 2016;2(12):2355-2364.[20] Gu X,Zheng Y,Cheng Y,et al.In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009;30(4):484-498.[21] Tamimi F,Le Nihouannen D,Bassett DC,et al. Biocompatibility of magnesium phosphate minerals and their stability under physiological conditions. Acta Biomaterialia.2011;7(6): 2678-2685.[22] Saris NEL,Mervaala E,Karppanen H,et al. Magnesium: an update on physiological, clinical and analytical aspects. Clinica Chimica Acta. 2000;294(1-2):1-26.[23] Kirkland NT,Birbilis N,Staiger MP.Assessing the corrosion of biodegradable magnesium implants: a critical review of current methodologies and their limitations. Acta Biomaterialia. 2012;8(3): 925-936.[24] White RE,Hartzell HC.Magnesium ions in cardiac function: regulator of ion channels and second messengers.Biochem Pharmacol. 1989; 38(6):859-867.[25] Gu XN,Zheng YF.A review on magnesium alloys as biodegradable materials. Front Mater Sci China.2010;4(2):111-115.[26] 郑玉峰,吴远浩.处在变革中的医用金属材料[J].金属学报, 2017,53(3): 257-297.[27] Unigovski Y,Eliezer A,Abramov E,et al. Corrosion fatigue of extruded magnesium alloys.Mater Sci Eng A. 2003;360(1-2):132-139.[28] Jafari S,Harandi SE,Raman RKS.A review of stress-corrosion cracking and corrosion fatigue of magnesium alloys for biodegradable implant applications. JOM. 2015;67(5):1143-1153.[29] Hänzi AC,Gerber I,Schinhammer M,et al.On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg-Y-Zn alloys.Acta Biomaterialia. 2010;6(5):1824-1833.[30] Li X,Chu C,Chu PK.Effects of external stress on biodegradable orthopedic materials: A review. Bioact Mater. 2016;1(1):77-84.[31] Jafari S,Raman RKS,Davies CHJ,et al. Stress corrosion cracking and corrosion fatigue characterisation of MgZn1Ca0.3 (ZX10) in a simulated physiological environment. J Mech Behav Biomed Mater. 2017;65:634-643.[32] Koo Y,Jang Y,Yun Y. A study of long-term static load on degradation and mechanical integrity of Mg alloys-based biodegradable metals. Mater Sci Eng B Solid State Mater Adv Technol. 2017;219:45-54.[33] Törne K,Örnberg A,Weissenrieder J.Influence of strain on the corrosion of magnesium alloys and zinc in physiological environments. Acta Biomaterialia.2017; (48): 541-550.[34] Zhou LF, Liu ZY,Wu W,et al.Stress corrosion cracking behavior of ZK60 magnesium alloy under different conditions. Int J Hydrogen Energ. 2017;42(41):26162-26174.[35] Choudhary L,Szmerling J,Goldwasser R,et al. Investigations into stress corrosion cracking behaviour of AZ91D magnesium alloy in physiological environment.Procedia Eng.2011;10(7):518-523.[36] Choudhary L,Raman RKS.Mechanical integrity of magnesium alloys in a physiological environment: Slow strain rate testing based study. Eng Fract Mech.2013;(103):94-102.[37] 宋仁国,杨芳儿,翁晓红,等.ZE41镁合金应力腐蚀开裂研究[C].全国氢脆与应力腐蚀及工程应用学术研讨会论文集,四川江油, 2007:33-36.[38] 王璐科,王振家,马全友,等.AZ91D 镁合金在两种盐溶液和空气中的一般腐蚀和应力腐蚀[J].腐蚀与防护, 2006,27(12):599-603.[39] 许越,李家学,李莎.镁合金应力腐蚀机理及其影响因素分析[J].材料科学与工艺,2008,16(3):314-318.[40] Chu CL,Han X,Bai J,et al. Surface modification of biomedical magnesium alloy wires by micro-arc oxidation.Trans Nonferrous Met Soc China. 2014;24(4):1058-1064.[41] Winzer N, Atrens A,Dietzel W,et al.Evaluation of the delayed hydride cracking mechanism for transgranular stress corrosion cracking of magnesium alloys. Mater Sci Eng A. 2007;466(1-2):18-31.[42] Winzer N,Atrens A,Song G,et al. A critical review of the stress corrosion cracking (SCC) of magnesium alloys. Adv Eng Mater. 2005;7(8):659-693.[43] Yang GF,Kim YC,Han HS, et al.In vitro dynamic degradation behavior of new magnesium alloy for orthopedic applications. J Biomed Mater Res B Appl Biomater. 2015;103(4):807-815.[44] Dong L,Song X,Ye Y,et al. Experimental research on degradation performance of magnesium alloy affected by mechanical environment.MATEC Web of Conferences. 2015;31:01006.[45] Zheng Y,Li Y,Chen J,et al.Effects of tensile and compressive deformation on corrosion behaviour of a Mg–Zn alloy. Corr Sci. 2015; (90):445-450.[46] Denkena B,Köhler J,Stieghorst J,et al. Influence of stress on the degradation behavior of Mg LAE442 implant systems. Procedia Cirp. 2013;5(2):189-195.[47] Wang H,Shi Z.In vitro biodegradation behavior of magnesium and magnesiumalloy. J Biomed Mater Res B Appl Biomater. 2011;98(2):203.[48] 劳永华,张文威,徐笑凡,等.AZ31镁合金在人工血浆中的动态降解行为[J].稀有金属材料与工程,2014,43(5):1242-1245.[49] Wang J,Giridharan V,Shanov V,et al.Flow-induced corrosion behavior of absorbable magnesium-based stents. Acta Biomaterialia. 2014; 10(12):5213-5223.[50] Wang J,He Y,Maitz MF,et al.A surface-eroding poly (1, 3-trimethylene carbonate) coating for fully biodegradable magnesium-based stent applications: toward better biofunction, biodegradation and biocompatibility. Acta Biomaterialia.2013;9(10):8678-8689.[51] Lévesque J,Hermawan H,Dubé D,et al. Design of a pseudo-physiological test bench specific to the development of biodegradable metallic biomaterials. Acta Biomaterialia.2008’4(2):284-295.[52] Jafari S,Raman RKS,Davies CHJ,et al.Corrosion fatigue of a magnesium alloy in modified simulated body fluid.Eng Fract Mech. 2015;137:2-11.[53] Gu XN,Zhou WR,Zheng YF,et al.Corrosion fatigue behaviors of two biomedical Mg alloys–AZ91D and WE43–in simulated body fluid. Acta Biomaterialia. 2010;6(12):4605-4613.[54] Bian D,Zhou W,Liu Y,et al.Fatigue behaviors of HP-Mg, Mg-Ca and Mg–Zn–Ca biodegradable metals in air and simulated body fluid. Acta Biomaterialia.2016; (41): 351-360.[55] Nie FL,Zheng YF,Wei SC,et al.In vitro corrosion, cytotoxicity and hemocompatibility of bulk nanocrystalline pure iron. Biomed Mater. 2010;5(6): 065015.[56] Li H, Zheng Y, Qin L.Progress of biodegradable metals Prog Nat Sci Mater Int.2014;24(5):414-422.[57] 郑玉峰,杨宏韬.血管支架用可降解金属研究进展[J].金属学报, 2017, 53(10):1227-1237.[58] Moravej M,Purnama A,Fiset M,et al.Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies. Acta Biomaterialia.2010;6(5):1843-1851.[59] Zhu S,Huang N,Xu L,et al.Biocompatibility of pure iron: in vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mater Sci Eng C.2009; 29(5):1589-1592.[60] Liu B, Zheng YF.Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomaterialia. 2011;7(3):1407-1420.[61] 吴远浩,周晓晨,李楠,等.可降解金属血管支架研究进展[J].中国材料进展, 2012,31(9):27-34.[62] Bowen PK,Shearier ER,Zhao S,et al.Biodegradable metals for cardiovascular stents: from clinical concerns to recent Zn‐Alloys. Adv Healthc Mater. 2016; 5(10):1121-1140.[63] Isotalo TM,Nuutine JP,Vaajanen A,et al.Biocompatibility properties of a new braided biodegradable urethral stent: a comparison with a biodegradable spiral and a braided metallic stent in the rabbit urethra. BJU Int.2006;97(4): 856-859.[64] Li HF,Xie XH,Zheng YF,et al.Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci Rep. 2015;5:10719.[65] Chen Y,Zhang W,Maitz MF,et al.Comparative corrosion behavior of Zn with Fe and Mg in the course of immersion degradation in phosphate buffered saline. Corr Sci. 2016;111:541-555. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Li Jun, Zuo Xinhui, Liu Xiaoyuan, Zhang Kai, Han Xiangzhen, He Huiyu, . Effect of over expression of miR-378a on osteogenic and vascular differentiation of bone marrow mesenchymal stem cell sheet [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 4939-4944. |
| [11] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [12] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [13] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [14] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [15] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||